What is Chrome Plating: Process, Types, Applications, and Benefits
Chrome plating transforms ordinary metal surfaces into durable, mirror-like finishes that capture attention and protect components. This electrochemical process deposits a thin layer of chromium onto metal substrates, creating surfaces that resist corrosion, reduce friction, and deliver exceptional hardness. The chrome plating process involves several critical steps, from thorough surface preparation to the final chromium deposition in specialized baths.
Essentially, two primary types of chrome plating exist: decorative chrome plating, which provides the brilliant finish found on automotive trim and bathroom fixtures, and hard chrome plating, which enhances industrial components with superior wear resistance. Furthermore, various specialized applications have emerged across industries, particularly in automotive, aerospace, medical, and consumer goods sectors.
The benefits of chrome plating extend beyond esthetics, as the technique significantly improves part longevity and performance in demanding environments. This comprehensive guide explores the chrome plating process, its diverse applications, and the advantages that make this metal finishing technique indispensable in modern manufacturing.
Understanding Chrome Plating Fundamentals
At the molecular level, chrome plating involves precise electrochemical reactions that bond chromium atoms to substrate materials. This metallic coating process depends on controlled electrical currents to deposit chromium from specialized solutions onto prepared surfaces. The resulting finish not only provides esthetic value but also substantial functional benefits.
Electrochemical Basis of Chrome Plating
Chrome plating operates on fundamental electrochemical principles. The process, also known as chromium electroplating, uses electricity to deposit a layer of chromium onto a metal or plastic surface. When an electrical current passes through a chromic acid solution, it causes chromium ions to bond to the surface of the part, creating a uniform chrome layer that enhances both appearance and performance.
The chemistry behind the process typically involves chromic acid (CrO₃) mixed with sulfuric acid. This extremely acidic bath (pH 0) serves as the electrolyte solution, with the ratio of chromic acid to sulfuric acid varying from 75:1 to 250:1 by weight. The process parameters, including bath temperature, current density, and duration, directly influence the quality, thickness, and properties of the final chrome coating.
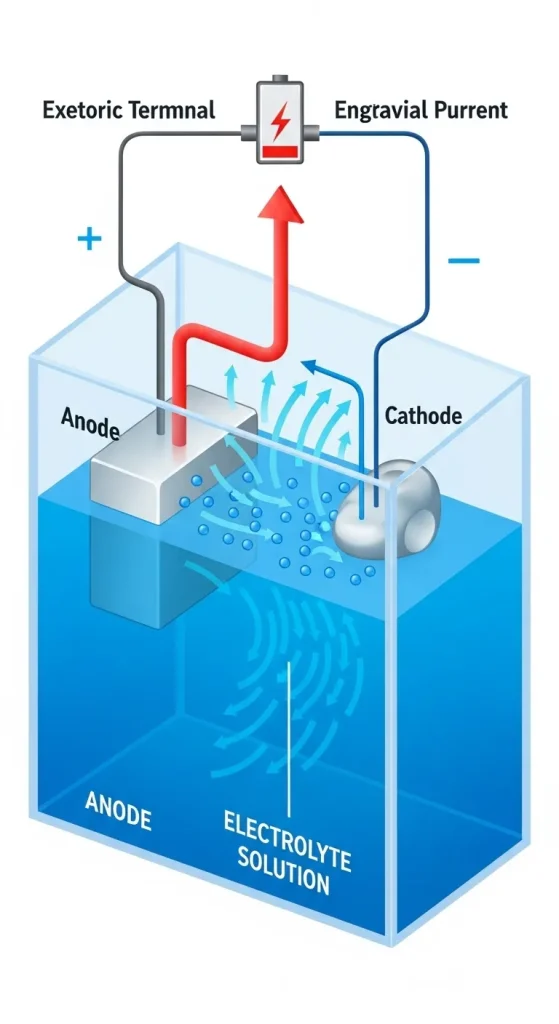
Role of Anode, Cathode, and Electrolyte
In the chrome plating setup, three critical components work together:
During operation, the electrical current causes chromium ions to move through the electrolyte and deposit onto the cathode surface. The current distribution significantly affects coating uniformity, with sharp edges potentially receiving five times higher current density than flat areas. This phenomenon, known as the “edge effect,” presents ongoing challenges in achieving consistent coating thickness on complex parts.
Difference Between Decorative and Hard Chrome
Two distinct approaches to chrome plating serve different purposes:
Decorative Chrome Plating
Hard Chrome Plating
The key difference lies not just in thickness but in primary purpose, decorative chrome creates visual appeal with some protective qualities, while hard chrome primarily enhances mechanical performance and durability for demanding industrial applications.
Step-by-Step Chrome Plating Process
Successful chrome plating requires meticulous attention to every step in the process sequence. Each phase directly influences the quality, durability, and appearance of the final chrome layer. From initial cleaning to final inspection, precision at each stage ensures optimal results for both decorative and functional applications.
Surface Cleaning and Degreasing Techniques
The chrome plating journey begins with thorough surface preparation. This initial step removes contaminants that would otherwise prevent proper adhesion of the chrome layer. Poor cleaning directly correlates with peeling, blistering, or complete plating failure.
Several cleaning approaches are employed depending on the substrate condition:
Following cleaning, the “water break test” becomes crucial, water should form a continuous sheet across the surface rather than beading up, indicating a truly clean surface.
Activation Bath Using Hydrochloric Acid
Prior to chrome application, the surface must be “activated” to ensure proper chrome adhesion. This step involves immersion in an acidic solution that etches the surface microscopically and removes any remaining oxides. A typical activation solution contains hydrochloric acid (also called muriatic acid) at 30-40% concentration. This strong acid creates a slightly roughened texture at the microscopic level, providing better mechanical interlock for the subsequent plating layers.
In some facilities, activation occurs in a chromic acid solution with reversed current, effectively etching the workpiece surface. This dual-purpose approach simultaneously removes scale and prepares the surface for plating.
Nickel Undercoating for Enhanced Adhesion
For optimal corrosion resistance and adhesion, many chrome plating operations apply a nickel layer before the chrome itself. This crucial step creates a smoother foundation and improves overall finish quality. Sulfamate nickel is frequently utilized as an undercoating prior to chrome application. This nickel layer protects the base metal from corrosion and enhances the final appearance of decorative chrome applications. The transfer time between nickel plating and chrome plating is critical, ideally under two minutes to prevent nickel passivation, which inhibits proper chrome adhesion. For extended transfer times, reactivation using acid solutions becomes necessary.
Chromium Deposition in Chromic Acid Bath
The actual chrome deposition occurs in a chromium bath containing chromic acid (CrO₃) and sulfuric acid in specific ratios, typically ranging from 75:1 to 250:1 by weight. This highly acidic bath (pH 0) requires precise temperature control.
Temperature requirements vary by application:
- Decorative plating: 35-45°C (100-110°F)
- Hard chrome plating: 50-65°C (120-150°F)
Current density significantly affects brightness and coverage, with higher densities requiring higher bath temperatures. A hexavalent chrome plating bath deposits approximately 0.001 inches (25 μm) per hour. One functional disadvantage of this process is poor “throwing power,” meaning chrome deposits unevenly, thicker on edges and thinner in inside corners and holes. To overcome this, parts may require over-plating and subsequent grinding to size.
Post-Plating Rinse and Polishing
After plating completion, thorough rinsing removes residual chemicals from the component’s surface. This “final bath” stage prevents unwanted residues from affecting the finish quality. For decorative applications, additional buffing and polishing may follow to achieve the characteristic mirror-like shine. Hard chrome applications might require precision grinding to achieve final dimensional tolerances.
Final Inspection and Quality Control
Quality control represents the final crucial step. Visual inspection identifies defects such as pitting, cloudiness, or peeling. Thickness measurement using X-ray fluorescence or magnetic induction confirms specification compliance. Adhesion testing, often involving thermal shock exposure or bend testing, verifies the durability of the chrome bond. For critical applications, corrosion resistance might be evaluated through salt spray testing (ASTM B117), while hardness testing verifies wear resistance properties.
Industry specifications govern acceptable quality standards, including AMS 2460 for aerospace applications and ASTM B177 for esthetic plating. Every chrome plated component must meet these exacting requirements before release for service.
Types of Chrome Plating and Their Use Cases
Chrome plating techniques vary considerably based on intended applications and desired characteristics. Each method produces specific surface properties that address unique industry needs, from extending component lifespan to creating distinctive esthetic finishes.
Hard Chrome Plating for Industrial Components
Hard chrome plating, often called industrial or functional chrome, creates a thick chromium layer measuring between 0.0002 to 0.020 inches that provides exceptional surface hardness (65-70 HRC). This industrial process delivers exceptional wear resistance, reduced friction, and moderate corrosion protection. Unlike decorative chrome, hard chrome prioritizes functionality over appearance.
Industrial applications relying on hard chrome include hydraulic cylinders, machine tooling, molds, and agricultural equipment components. The coating excels in high-stress environments where mechanical durability remains paramount. Moreover, hard chrome can repair worn or mis-machined parts, restoring original dimensions without manufacturing replacement components.
Decorative Chrome for Consumer Products
Decorative chrome creates the familiar mirror-like finish found on automotive trim, bathroom fixtures, and household appliances. This ultra-thin layer (sometimes as thin as 50 millionths of an inch) is applied over nickel plating to achieve a brilliant, reflective appearance. The nickel underlayer provides the primary corrosion protection, while the chrome delivers scratch resistance and prevents tarnishing.
In contrast to hard chrome, decorative plating prioritizes esthetics and employs different bath formulations and processing parameters to achieve optimal brightness and reflectivity.
Thin Dense Chrome for Precision Applications
Thin Dense Chrome (TDC) provides a compromise between decorative and hard chrome properties. With thicknesses ranging from 0.0001″ to 0.0002″, this specialized coating maintains tight dimensional tolerances while delivering excellent wear and corrosion resistance. The dense structure, created through specific bath chemistry and application techniques, yields a coating free of micro cracks. Engineers choose TDC for applications requiring precise dimensions, as the coating adheres without edge build-up or distortion. Food and medical equipment frequently utilize TDC since it resists chipping, peeling, or flaking, critical for contamination prevention.
Satin and Black Chrome for Esthetic Finishes
Satin chrome delivers a matte, non-reflective finish that offers a subtle, elegant appearance compared to traditional bright chrome. This effect is achieved by brushing the base metal before applying a standard chrome layer.
Black chrome plating produces a dark, sophisticated appearance through specialized bath chemistry that incorporates additives to darken the final color. Originally developed in the 1960s, modern black chrome processes use trivalent chromium alloyed with other metals to achieve smoother, more reflective dark finishes without requiring post-dip treatments.
Micro-Cracked and Micro-Porous Chrome for Lubrication Retention
Micro-cracked chrome contains a network of fine, controlled cracks formed during the plating process. These cracks prevent larger, catastrophic cracking under stress by distributing strain evenly across the surface. Additionally, the micro-cracks create small reservoirs that retain lubricants, enhancing performance in applications like hydraulic cylinders and industrial machinery.
Micro-porous chrome differs by incorporating millions of tiny inert particles in the nickel underlayer. These particles create microscopic voids in the chrome, dispersing corrosion mechanisms across the surface and enhancing electrochemical protection. This triplex nickel structure significantly improves corrosion resistance compared to standard duplex nickel systems.
Applications Across Industries
Chrome plating versatility extends across numerous industries, providing both functional and esthetic benefits for specialized components. Each sector leverages specific chrome plating variations to address unique operational demands.

Automotive: Bumpers, Exhausts, and Trim
The automotive industry relies heavily on chrome plating for exterior components like bumpers, grilles, and trim elements where it enhances visual appeal while providing corrosion protection. Vehicle manufacturers apply chrome to exhaust systems, door handles, and window trim to improve both durability and esthetics. Engine components benefit from hard chrome’s friction-reducing properties, ultimately extending component lifespan. Wheel applications introduce a luxurious appearance while simultaneously protecting against road salt and harsh weather conditions.
Aerospace: Landing Gear and Actuators
Aerospace applications demand exceptional performance under extreme conditions. Chrome plating protects landing gear components, turbine blades, and hydraulic systems from wear and corrosion. Aircraft engine parts consequently gain resistance to friction, stress, and high temperatures. Hard chrome plating enables aircraft components to withstand pressures up to 30,000 psi, thereby ensuring operational safety. The coating’s ability to minimize damage from drilling and heat generation makes it indispensable for flight-critical systems.
Medical: Surgical Tools and Dental Equipment
Medical device manufacturers utilize chrome plating on surgical instruments, ensuring easy sterilization and preventing corrosion. Dental equipment benefits from chrome’s durability and hygienic properties, withstanding repeated cleaning cycles. Specialized medical-grade chromium coatings like MEDCOAT 2000™ improve component performance by decreasing wear effects associated with intensive use. Surgical blades, bone drills, and cannulas receive biocompatible chrome finishes that meet ISO 10993-1 certification requirements.
Oil & Gas: Valves and Pipeline Fittings
The oil and gas sector employs chrome plating on pumps, valves, and pipeline fittings to withstand corrosive chemicals and extreme temperatures. Hard chrome provides a protective barrier that increases component hardness and heat resistance. Ball valves, hydraulic rams, and crankshafts in drilling equipment receive chrome plating to prevent damage and extend operational life. For ultimate protection in aggressive environments, electroless nickel is occasionally applied beneath hard chrome.
Consumer Goods: Faucets, Utensils, and Fixtures
Decorative chrome enhances both appearance and longevity of everyday items including kitchen utensils, faucets, and household fixtures. Furniture accents, door handles, and appliance components benefit from chrome’s reflective finish and scratch resistance. Chrome-plated kitchen tools gain improved corrosion and heat resistance, ensuring durability for daily use. The ease of cleaning and sanitizing these chrome surfaces makes them ideal for food preparation environments. If you’re interested in other surface finishing techniques, check out our Hydro Dipping page to explore unique water-transfer designs and film options.
Benefits and Limitations of Chrome Plating
Beyond mere esthetics, chrome plating delivers substantial functional benefits alongside notable drawbacks. Understanding these factors helps industries determine whether this coating method suits their specific requirements.
Corrosion Resistance and Surface Hardness
Chrome plating forms a protective barrier against oxidation, effectively preventing rust formation on metal surfaces. This protection extends to various chemical environments, as chrome resists damage from citric acids, nitric acids, and other corrosive substances. The coating withstands weather extremes, shielding components from damage due to temperature fluctuations and varying humidity levels.
Notably, hard chrome exhibits remarkable hardness, typically measuring between 65-70 HRC (940-1210 HV). This exceptional hardness makes chrome-plated surfaces ideal for applications requiring resistance to physical wear and abrasion.
Friction Reduction and Wear Protection
Perhaps the most significant engineering advantage of chrome plating is its outstanding lubricity.. This property minimizes friction between moving parts, substantially reducing wear over time. Chrome’s low coefficient of friction prevents galling, a form of wear caused by adhesion during sliding motion between materials.
In dry conditions, chromium on hard steel has a coefficient of friction of 0.15, compared to 0.42 for unplated hard steel. This friction reduction allows for more efficient moving parts and prevents overheating.
Health Concerns with Hexavalent Chromium
Despite its benefits, hexavalent chromium (Cr⁶⁺) poses serious environmental and health risks. It ranks as the second most potent toxic air contaminant identified by California, approximately 500 times more toxic than diesel exhaust. Long-term exposure increases lung cancer risk and can cause nasal and sinus cancers. Additional health effects include respiratory irritation, nasal ulceration, kidney and liver damage, skin irritation, and eye damage.
Cost Factors: Preparation, Thickness, and Complexity
Chrome plating expenses primarily stem from three factors: process chemicals/equipment, labor costs, and electricity consumption. Substrate preparation represents one of the largest cost components, thorough cleaning, defect removal, and pre-plating polishing significantly impact the final quality. Bath chemistry maintenance affects both cost and quality; inferior plating shops often allow electrolyte baths to degrade, causing inconsistent deposits.
Conclusion
Chrome plating stands as a remarkable metal finishing technology that transforms ordinary surfaces into durable, gleaming components across countless applications. Throughout this guide, we have explored the intricate electrochemical process that bonds chromium atoms to substrate materials, creating finishes that last for decades under demanding conditions. Certainly, the distinction between decorative and hard chrome serves different purposes, one delivering mirror-like esthetics while the other provides industrial-grade performance.
The meticulous step-by-step process reveals why quality chrome plating demands expertise and precision. Each phase from initial cleaning through final inspection directly affects the durability and appearance of the finished product. Additionally, specialized variations like thin dense chrome, black chrome, and micro-cracked chrome address specific industry needs beyond standard applications.
