Understanding UV Curing: The Basics of Light-Based Manufacturing
UV curing transforms manufacturing by processing materials in seconds instead of hours. The technology fully cures most UV light-curable materials in just 1-30 seconds. This breakthrough has changed modern production lines completely. The technology emerged in the 1960s and has simplified processes in manufacturing sectors of all types.
The UV curing process works differently than conventional methods. Traditional approaches rely on solvent evaporation that shrinks coatings by more than 50% and creates environmental pollutants. UV cure takes a different approach by triggering a photochemical reaction that causes solutions to polymerize. Many industries now use this technology, including automotive, appliance, aerospace, medical device, telecommunications, military, consumer electronics, and graphics arts.
UV curing coating applications provide superior scratch resistance, chemical resistance, and stronger adhesion, qualities that are equally valuable in processes like hydro dipping and chrome plating. In hydro dipping, UV-cured topcoats protect printed films from wear and fading, while in chrome plating, UV coatings enhance surface durability and shine without the need for lengthy drying times. This piece explains what UV light curing is, how it works, and why it has become a crucial technology in modern surface finishing and manufacturing industries.
What is UV Curing and How Does It Work?
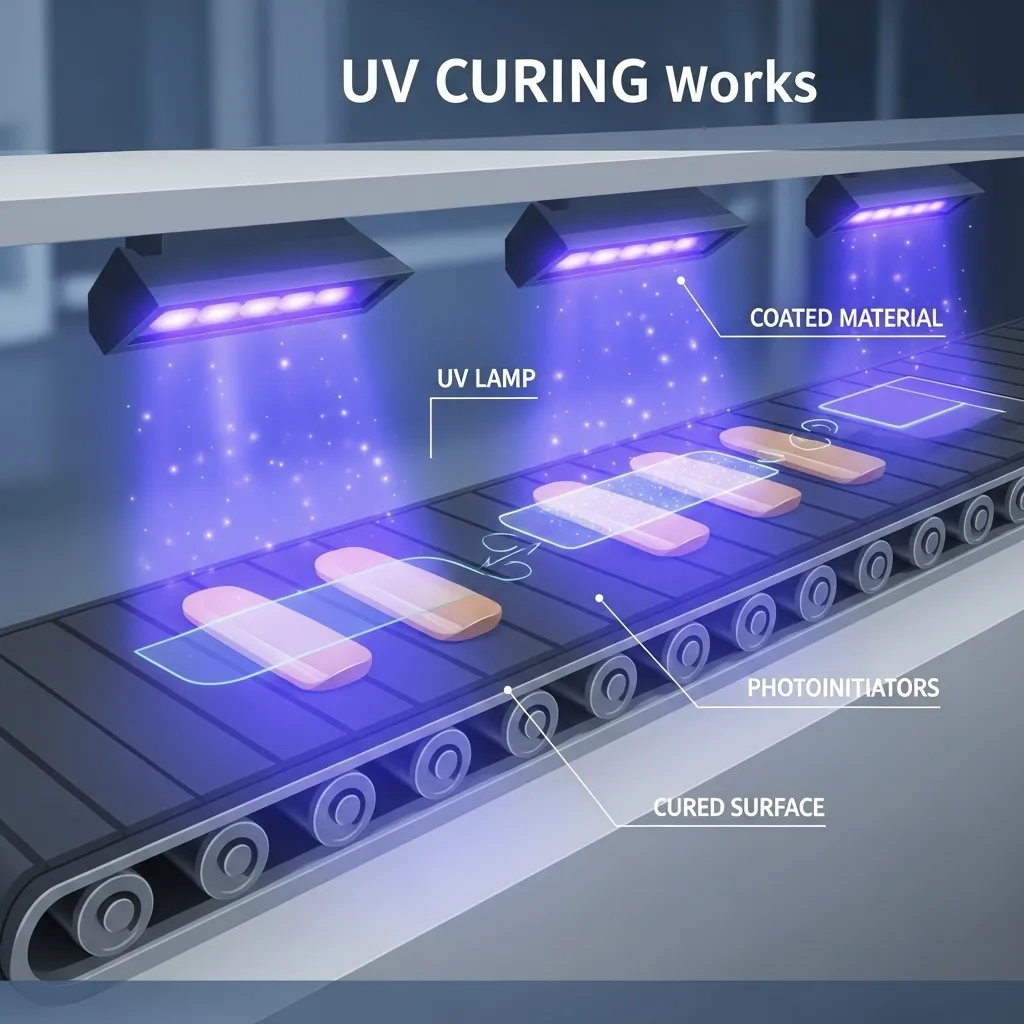
UV curing works as a photochemical process that uses ultraviolet light to turn liquid materials into solid polymers instantly. This process differs from traditional methods that depend on evaporation or heat, as UV curing creates a crosslinked network through radical or cationic polymerization.
Three key components work together to make this happen. Photoinitiators serve as special molecules that absorb UV energy and split apart to create reactive species. The base material consists of monomers and oligomers. A UV light source delivers the energy needed for the process.
UV light exposure causes photoinitiators to set off a chain reaction that makes monomers and oligomers cross-link faster. The material hardens in seconds or even milliseconds through this polymerization. Success depends on specific wavelengths (200-480 nm), proper light intensity, and enough exposure time.
Traditional thermal drying shrinks coatings by more than 50% as solvents evaporate. UV curing keeps 100% of the original thickness because nothing evaporates. On top of that, it substantially reduces environmental hazards compared to traditional drying methods that release volatile organic compounds.
The process’s speed stands out – UV curing changes materials almost instantly, while heat-based methods can take hours or days. This quick transformation lets you handle, package, or process materials right away.
Core Components of a UV Curing System
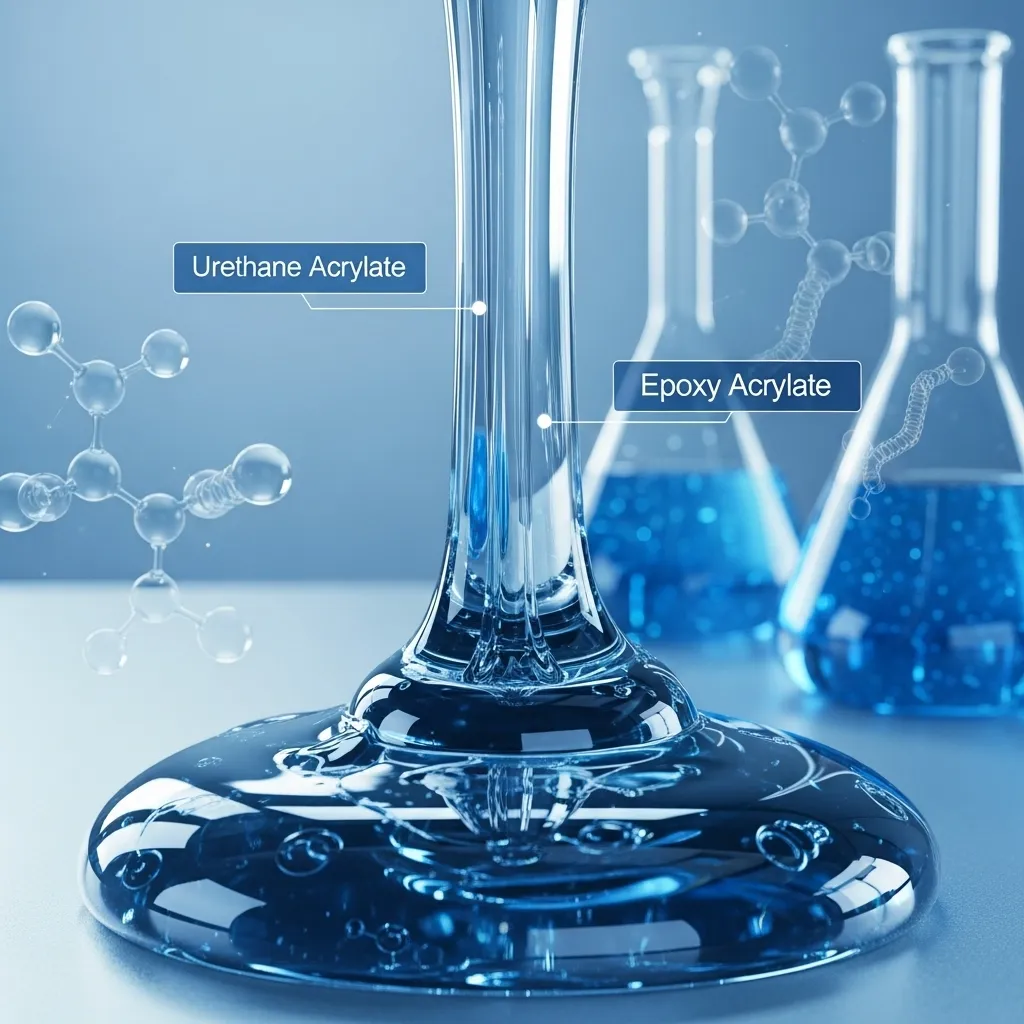
Oligomers
Oligomers serve as the foundation of every UV-curable formulation. They determine the key physical properties of the cured material, such as hardness, flexibility, and scratch resistance. These low molecular weight polymers come in different types, each offering distinct benefits.
Urethane acrylates, for example, add toughness and flexibility, while epoxy acrylates are known for their excellent chemical resistance. The careful selection of oligomers ultimately defines how the finished coating or adhesive performs under various conditions.
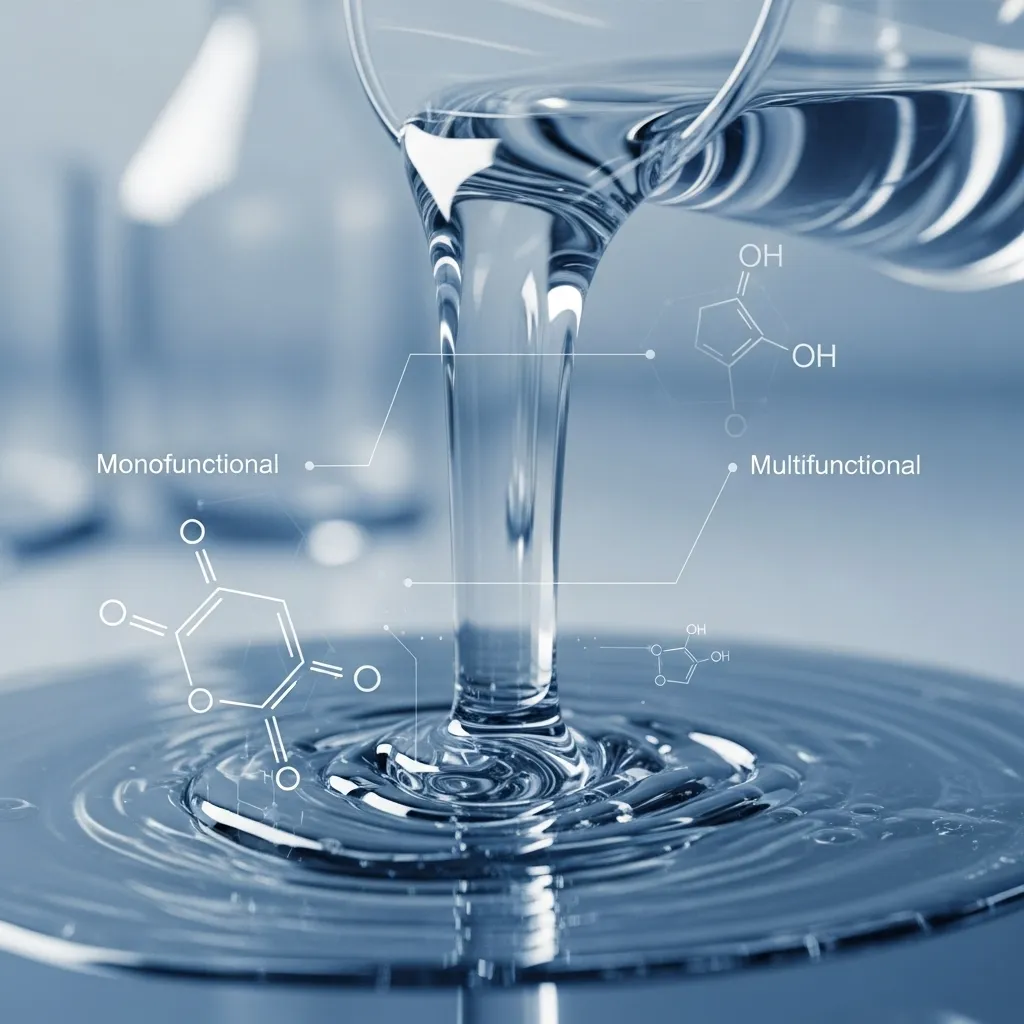
Monomers
Monomers play a vital role in reducing viscosity, which makes the formulation easier to apply during the coating or printing process. Once exposed to UV light, these monomers react and become part of the final solid material.
Monofunctional monomers have one reactive site and contribute flexibility to the cured product, whereas multifunctional monomers contain multiple reactive sites that create crosslinks between oligomer chains, resulting in higher strength and rigidity. The balance between these two types of monomers determines the overall performance of the final cured layer.
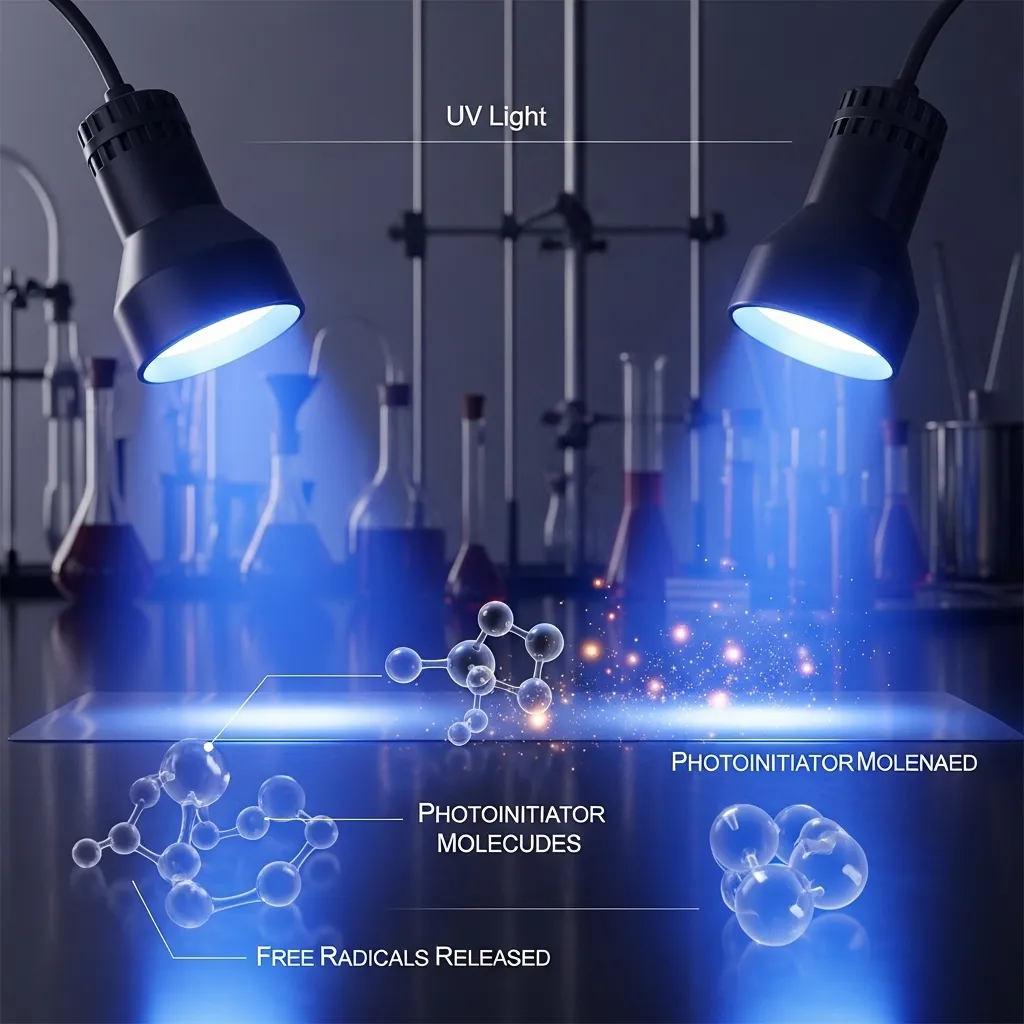
Photoinitiators
Photoinitiators are the driving force behind the curing reaction. Although they make up only a small percentage of the overall formulation, their role is critical. These specialized molecules absorb ultraviolet light and release free radicals that initiate the polymerization process.
Without photoinitiators, the curing reaction would not begin, no matter how intense the UV exposure. Their proper selection ensures efficient light absorption and optimal curing speed, which directly impacts production efficiency and product quality.

Additives
Additives are used to fine-tune the formulation and enhance specific properties of the cured material. Stabilizers help prevent premature curing during storage, while pigments and defoamers improve the visual appearance and surface quality. Adhesion promoters ensure that the coating adheres well to different substrates, improving durability and performance. Although additives make up a smaller portion of the mix, they significantly influence both processing and end-use characteristics.
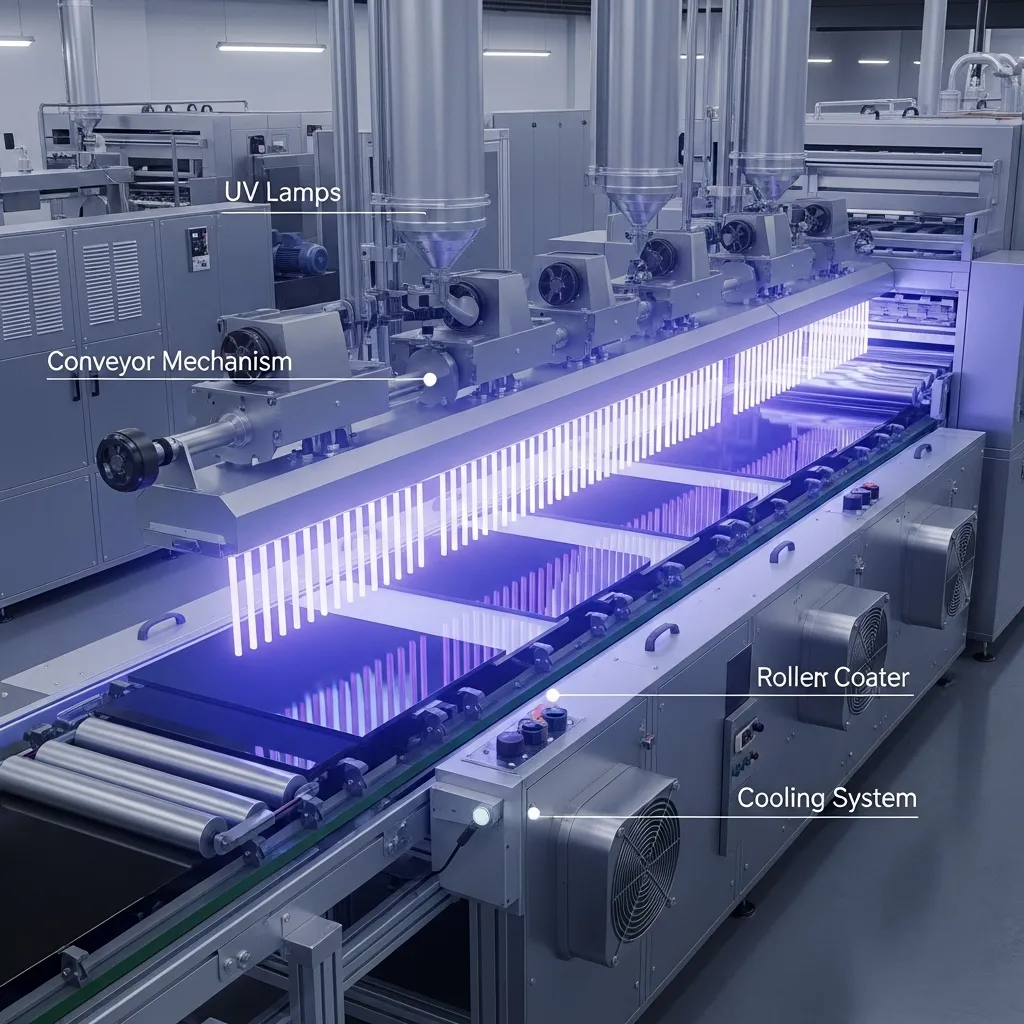
Equipment
The equipment used in a UV curing system is just as important as the chemistry. A typical setup includes a conveyor mechanism that moves products at a controlled speed, application equipment like roller coaters to apply the liquid evenly, and UV curing stations that house the lamps, reflectors, and cooling systems. The lamps provide the UV energy needed to activate the photoinitiators, while reflectors focus the light for maximum efficiency. Cooling systems maintain optimal temperatures to prevent overheating. Together, these components work seamlessly to transform a liquid formulation into a fully cured, solid material within seconds.
Types of UV Curing Mechanisms and Light Sources
UV curing works through two main photopolymerization mechanisms. A chain reaction transforms acrylate resins through free radical polymerization when photoinitiators create reactive species under UV exposure. This mechanism serves as the foundation for most UV curing applications but runs into oxygen inhibition issues. Cationic polymerization, which Crivello developed in 1977, takes a different approach. It cures epoxy resins through acid generation and continues working even after UV exposure stops—a process known as “dark reaction”.
These reactions need three different light sources. Medium-pressure mercury vapor lamps dominate the industry and work at temperatures between 600-900°C. They produce broad-spectrum UV output (200-600 nm) and deliver high power densities around 100 W/cm². Manufacturers can fine-tune the spectral output by adding metal halides to match specific applications.
Microwave-powered UV systems bring significant advantages to the table. These electrodeless lamps last much longer than traditional arc lamps. Their warranty covers 6000-8000 hours compared to arc lamps’ 1000-2000 hours. They also reach full power quickly, needing only about 15 seconds.
UV LED technology stands out as the latest game-changer. These systems mainly emit UVA wavelengths (365-405 nm). LEDs switch on and off instantly without any warm-up time. Their useful life stretches beyond 20,000 hours, which makes them increasingly attractive despite higher original costs.
Conclusion
UV curing is a game-changing advancement in manufacturing that’s changing production processes in industries of all types. This piece shows how the photochemical process turns liquid materials into solid polymers using ultraviolet light instantly. The process takes seconds instead of hours, so it saves valuable production time while keeping 100% of the original coating thickness.
The environmental advantages of UV curing are worth getting into. The process substantially cuts down volatile organic compounds compared to regular methods that use solvent evaporation. The quick transformation lets you handle and process materials right away – a huge plus for production speed.
The process needs two key components to work properly. The chemistry relies on photoinitiators, oligomers, and monomers that create the right material properties. The equipment then delivers exact UV energy to start the curing reaction. These elements need to work together perfectly.
Free radical and cationic polymerization mechanisms are a great way to get flexibility for different uses. You can pick between traditional mercury vapor lamps, microwave-powered systems, or the newer UV LED technology based on what you need. UV curing has without doubt proven its worth, with companies in automotive, aerospace, medical, and consumer electronics industries using it widely.
The technology keeps getting better, especially when you have more efficient UV LED systems coming up. We expect more manufacturers worldwide to adopt this process. UV curing technology gives you the perfect mix of speed, efficiency, and environmental responsibility – everything modern manufacturing just needs.
